Formaldehyde & derivates
Essential for the chemical industry

For more than 25 years Pörner Grimma has been liaising with renowned European licensors and experienced partners to build plants for the formaldehyde product family as an EPCM contractor.
In close cooperation with Dynea, the longtime licensing partner, the silver catalyst process for the production of formalin has been constantly improved to become the best of its kind. Technical and economic comparisons of several international clients as well as the experience from joint design and construction of more than 10 plants in the last years prove this.
As an EPC contractor Pörner sells, designs and builds plants of the formaldehyde technology family. Together with international licensors and technology partners Pörner Grimma can offer the following production plants with offsite, utilities and infrastructure units:
Formaldehyde is one of the most important organic raw materials in the chemical industry. Formaldehyde is an important raw material for the chemical industry and the basis for many industrial products. More than 50 branches of industry using formaldehyde and its derivatives, as adhesives, resins, and in many other applications. The global annual consumption of formaldehyde is about 41 million tons. 51% of them are made in China.
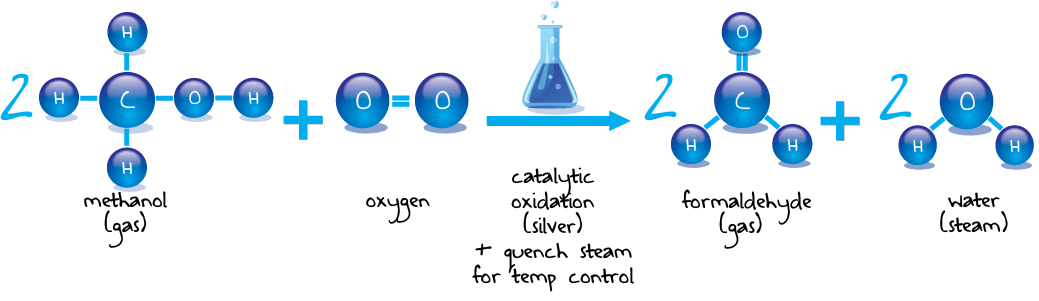
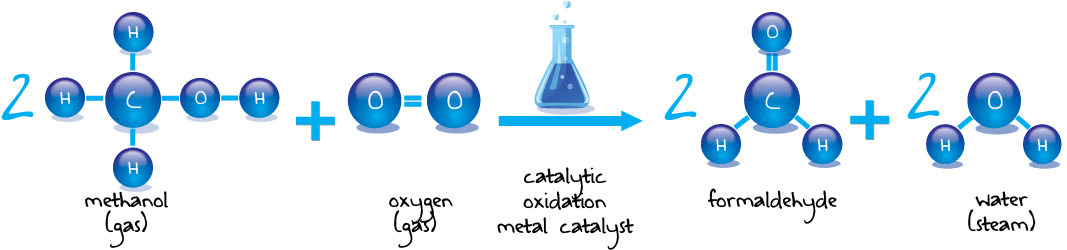
Pörner Grimma is not only a specialist in the formaldehyde method, but also for process applications of formaldehyde derivatives. Technically formaldehyde can be produced by the catalytic oxidation of methanol. These are, two methods, the silver and the metal oxide method.
Although the catalyst has to be changed frequently in the silver method, but the aqueous formaldehyde solution is of higher quality because the formic acid content is lower.
The Silver Catalyst process is the most safe process of formaldehyde production. Formaldehyde concentration and residue methanol produced with the Silver Catalyst Process is as good as from best metal oxide process. Less formic acid and no caustic soda and antifoam agent makes the product more versatile for all formulations and customers.
There is also no hot oil used in this process, just water/steam and this reduces the fire risk. There is no oxygen in the absorber, this not only reduces the fire risk but improves the product quality as well.
The catalyst can be removed without dust and waste in a few hours. Re-catalization can take less than 24 hours so only small holding tanks are necessary to serve customers during re-catalization.
The investment costs for the Silver Catalyst Process are similar compared to the Metal Oxide Process but there are other advantages to this system:
These factors more than compensate for the slightly higher methanol consumption compared to the Metal Oxide Process.
The Metal Oxide Process yields better quantities of formaldehyde therefore there is slightly less consumption of methanol. It also has similar investment costs to the Silver Catalyst Process.
Our process also allows the free choice of catalyst supplier as Pörner and its licensor are independent companies with the client’s wishes at heart. The final decision on which process to choose depends on the individual client and their needs.
Factors that influence this decision include costs of raw materials, utility costs, catalyst costs and safety and operation aspects etc.
Dynea, one of the world's leading manufacturers of formaldehyde, has decades of experience and has developed its own proprietary process, the silver catalyst-formaldehyde technology - fasil ™.
fasil ™ is the safest and most environmentally friendly formaldehyde process on the market. Catalyst research and development as well as feedback from the operational area guarantee formaldehyde production at the lowest total cost of ownership. fasil ™ is the preferred option for first-class customers looking for a safe, bespoke, low-cost concentrate-formaldehyde process.

Formaldehyde is an important raw material for the chemical industry and is used to manufacture many industrial and consumer products. Over 50 branches of industry now use formaldehyde and its derivatives in glues and resins and many other industrial applications. Because Pörner Grimma is the specialist in process design for Formaldehyde, we also specialize in the process design for formaldehyde derivatives. These derivatives are used for many different applications across industry and households.
They include:
Silver catalyst process
Metal oxide process
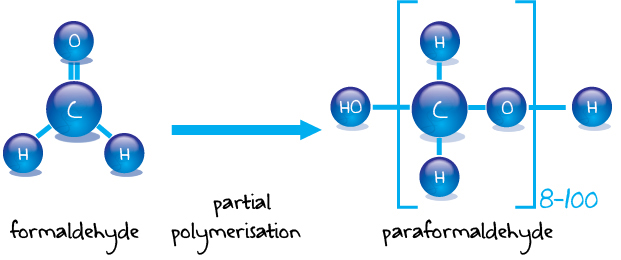
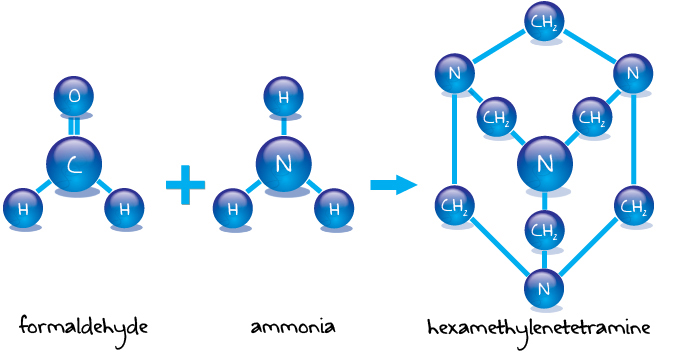
Hexamine liquid phase process
Hexamine gaseous phase process
Ethanol process:
Glues and resin plants designed and supplied by Pörner are tailor adapted and optimized production units for a broad range of products.
The product groups that Pörner caters for are: